Enzyme Activity Measurement for Oxidoreductases Using Spectrophotometric Assays
Oxidoreductases are a class of enzymes that catalyze the transfer of electrons from one molecule to another. The molecule that gives the electrons are called the electron donor or reductant, and the one receiving the electrons are called the electron acceptor or oxidant. Oxidoreductase enzymes play an important role in metabolic process. They can be found in both aerobic and anaerobic pathways, including glycolysis, TCA cycle, oxidative phosphorylation, and amino acid metabolism. To complete their functions, the oxidoreductase enzymes utilize redox cofactors. Common redox cofactors include FAD (flavin adenine dinucleotide), Fd (ferredoxin) FMN (flavin mononucleotide), NAD (nicotinamide adenine dinucleotide), NADP (nicotinamide adenine dinucleotide phosphate), Coenzyme B, Coenzyme Q, etc. For example, the enzyme glyceraldehydes-3-phosphate dehydrogenase reduce NAD+ to NADH in glycolysis. While NADH is re-oxidized to NAD+ in the oxidative phosphorylation pathway to maintain the proper re-dox state of the cell. Pyruvate is produced during glycolysis, which is then reduced to lactate through anaerobic glycolysis using NADH as the reductant. The redox cofactors bear one or more aromatic or unsaturated cyclic chemical groups in their structures. As a result, the cofactors strongly absorb UV or visible light in the reduced or oxidized form, or both. This makes the redox cofactors perfect reporters to the spectrophotometric enzyme assays.
Oxidorecuctases can be categorized into different subtypes, including oxidases and dehydrogenases. Oxidase enzymes use the molecular oxygen as the acceptor of hydrogen or electrons. To the contrary, dehydrogenase enzymes transfer hydrogen to NAD, NADP, or a flavin in order to oxidize a substrate. Other oxidoreductases include reductases, peroxidases, hydroxylases, and oxygenases. Reductases could also be oxidases since most redox reactions are reversible. The direction of the redox reaction depends both thermodynamics and kinetics. Peroxidases catalyze the decomposition of hydrogen peroxide, which is a natural defensive process against environmental invaders. Hydroxylases incorporate hydroxyl groups into organic compounds, while oxygenases add oxygen from molecular oxygen to the substrates.
Creative Enzymes proudly offers spectrophotometric enzyme assays in quantification of activity levels for all types of oxidoreductase enzymes:
- Alcohol oxidoreductases (EC 1.1, oxidoreductases that act on the CH-OH group of donors)
- Oxidoreductases acting on the aldehyde or oxo group of donors (EC 1.2)
- CH–CH oxidoreductases (EC 1.3, oxidoreductases that act on the CH-CH group of donors)
- Oxidoreductases that act on the CH-NH2 group of donors (EC 1.4, amino acid oxidoreductases, monoamine oxidases)
- Oxidoreductases that act on CH-NH group of donors (EC 1.5)
- Oxidoreductases that act on NADH or NADPH (EC 1.6)
- Oxidoreductases that act on other nitrogenous compounds as donors (EC 1.7)
- Oxidoreductases that act on a sulfur group of donors (EC 1.8)
- Oxidoreductases that act on a heme group of donors (EC 1.9)
- Oxidoreductases that act on diphenols and related substances as donors (EC 1.10)
- Peroxidases (EC 1.11, oxidoreductases that act on peroxide as an acceptor)
- Oxidoreductases that act on hydrogen as donors (EC 1.12)
- Oxygenases (EC 1.13), oxidoreductases that act on single donors with incorporation of molecular oxygen
- Oxidoreductases that act on paired donors with incorporation of molecular oxygen (EC 1.14)
- Oxidoreductases that act on superoxide radicals as acceptors (EC 1.15)
- Oxidoreductases that oxidize metal ions (EC 1.16)
- Oxidoreductases that act on CH or CH2 groups (EC 1.17)
- Oxidoreductases that act on iron-sulfur proteins as donors (EC 1.18)
- Oxidoreductases that act on reduced flavodoxin as a donor (EC 1.19)
- Oxidoreductases that act on phosphorus or arsenic in donors (EC 1.20)
- Oxidoreductases that act on X-H and Y-H to form an X-Y bond (EC 1.21)
- Other oxidoreductases (EC 1.97)
As a large and important family of enzymes, oxidoreductases are used widely in the pharmaceutical industry for syntheses of amino acids, steroids, and other molecules; in the chemical industry for production of specialty chemicals; in clinical diagnosis and other analytical applications; and in other applications such as material modification and pollution control.
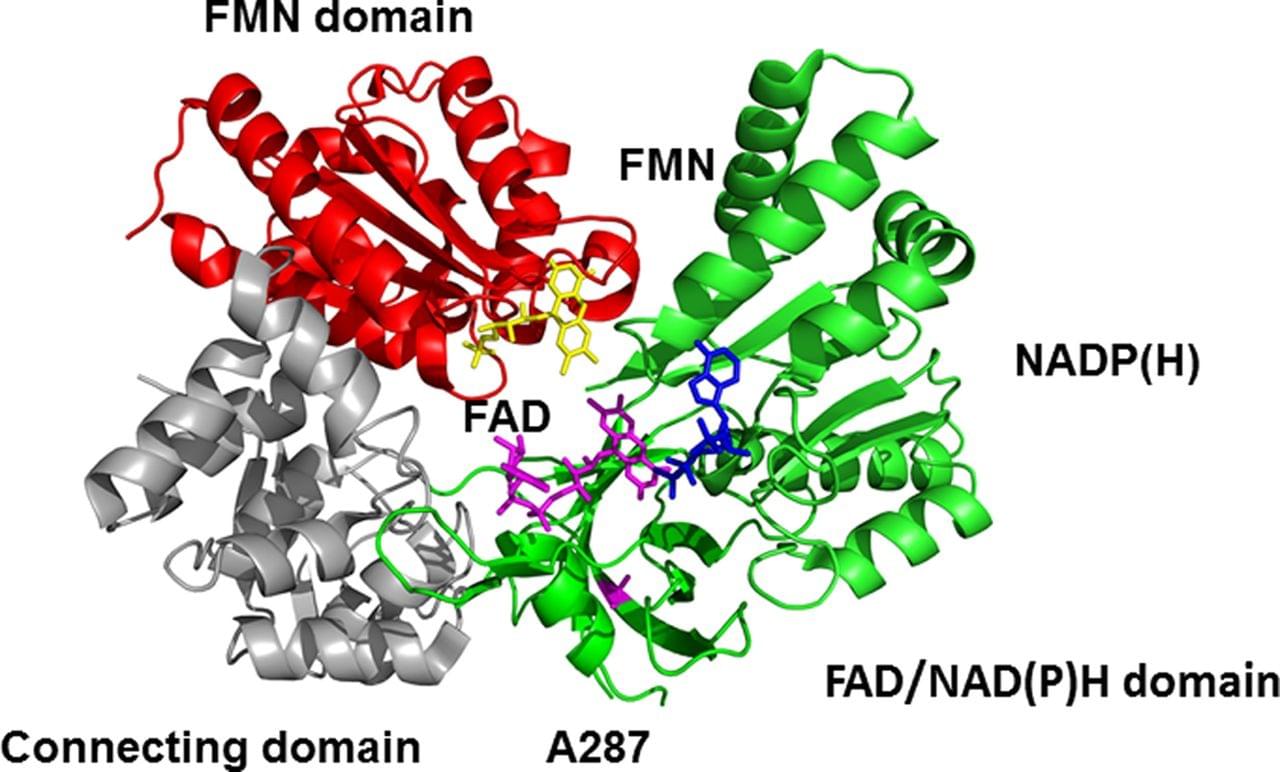 Figure: The structure of a human cytochrome P450 reductase (EC 1.6.2.4) showing cofactors NADPH (blue) and FAD (magenta), and a prosthetic group FMN (yellow).
Figure: The structure of a human cytochrome P450 reductase (EC 1.6.2.4) showing cofactors NADPH (blue) and FAD (magenta), and a prosthetic group FMN (yellow).
Reference: Jin, Y.; Chen, M.; Penning, T.; Miller, W. Biochem. J. 2015, 468 (1), 25.
Related Services
Enzyme Activity Measurement for Oxidoreductases Acting on Carbon Using Spectrophotometric Assays
Enzyme Activity Measurement for Oxidoreductases Acting on Nitrogen and Sulfur Using Spectrophotometric Assays
Enzyme Activity Measurement for Oxidoreductases Interacting with Inorganics