Effect of Temperature on Enzymatic Reaction
As temperature increases so do the rate of enzyme reactions. A ten degree centigrade rise in temperature will increase the activity of most enzymes by 50% to 100%. Variations in reaction temperature as small as 1 or 2 degrees may introduce changes of 10% to 20% in the results. This increase is only up to a certain point until the elevated temperature breaks the structure of the enzyme. Once the enzyme is denatured, it cannot be repaired. As each enzyme is different in its structure and bonds between amino acids and peptides, the temperature for denaturing is specific for each enzyme. Because most animal enzymes rapidly become denatured at temperatures above 40°C, most enzyme determinations are carried out somewhat below that temperature.
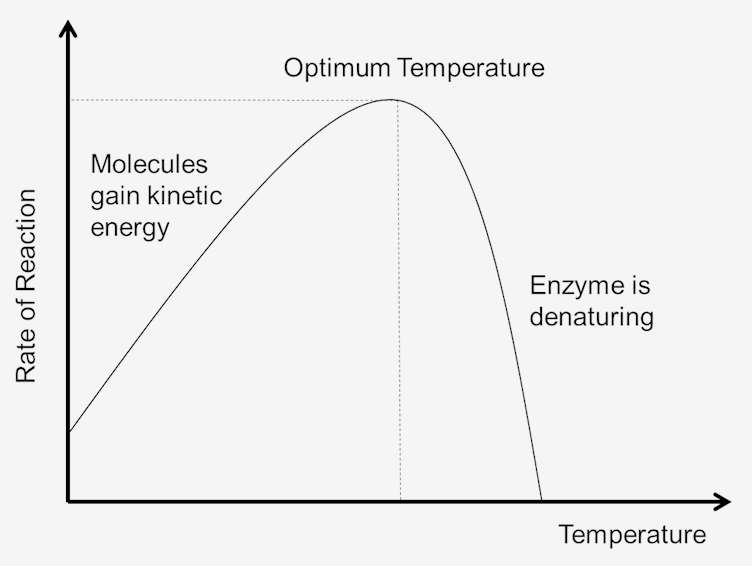 Figure 1. Effect of temperature on reaction rate.
Figure 1. Effect of temperature on reaction rate.
Over a period of time, enzymes will be deactivated at even moderate temperatures. Storage of enzymes at 5°C or below is generally the most suitable. Lower temperatures lead to slower chemical reactions. Enzymes will eventually become inactive at freezing temperatures but will restore most of their enzyme activity when temperatures increase again, while some enzymes lose their activity when frozen.
Kinetic Energy and Internal Energy
The temperature of a system is to some extent a measure of the kinetic energy of the molecules in the system. Collisions between all molecules increase as temperature increases. This is due to the increase in velocity and kinetic energy that follows temperature increases. With faster velocities, there will be less time between collisions. This results in more molecules reaching the activation energy, which increases the rate of the reactions. Since the molecules are also moving faster, collisions between enzymes and substrates also increase. Thus the lower the kinetic energy, the lower the temperature of the system and, likewise, the higher the kinetic energy, the greater the temperature of the system.
As the temperature of the system is increased, the internal energy of the molecules in the system will increase. The internal energy of the molecules may include the translational energy, vibrational energy and rotational energy of the molecules, the energy involved in chemical bonding of the molecules as well as the energy involved in nonbonding interactions. Some of this heat may be converted into chemical potential energy. If this chemical potential energy increase is great enough some of the weak bonds that determine the three-dimensional shape of the active proteins may be broken. This could lead to thermal denaturation of the protein and thus inactivate the protein. Thus too much heat can cause the rate of an enzyme-catalyzed reaction to decrease because the enzyme or substrate becomes denatured and inactive.
Optimum Temperature
Each enzyme has a temperature range in which a maximal rate of reaction is achieved. This maximum is known as the temperature optimum of the enzyme. The optimum temperature for most enzymes is about 98.6 degrees Fahrenheit (37 degrees Celsius). There are also enzymes that work well at lower and higher temperatures. For example, Arctic animals have enzymes adapted to lower optimal temperatures; animals in desert climates have enzymes adapted to higher temperatures. However, enzymes are still proteins, and like all proteins, they begin to break down at temperatures above 104 degrees Fahrenheit. Therefore, the range of enzyme activity is determined by the temperature at which the enzyme begins to activate and the temperature at which the protein begins to decompose.
Related Services
Enzyme Kinetics
Enzymology Assays
To discuss more service details, please contact us.