Ferroxidases/laccases
Ferroxidase also known as Fe(II):oxygen oxidoreductase is an enzyme that catalyzes the oxidization of iron II to iron III:
4 Fe2+ + 4 H+ + O2 = 4 Fe3+ + 2H2O
Human genes encoding proteins with ferroxidase activity include ceruloplasmin, ferritin heavy chain, ferritin, mitochondrial, and hephaestin.
1. CP – Ceruloplasmin
Ceruloplasmin (CER), also known as copper oxidase, is a copper-containing α2 glycoprotein with a molecular weight of about 120,000 to 160,000, which is difficult to purify. It is a single-chain polypeptide containing 6-7 copper atoms per molecule. It is blue due to copper and contains about 10% sugar. The terminal sialic acid is linked to the polypeptide chain and has genetic polymorphism. Its role is to regulate the distribution of copper in various parts of the body, to synthesize copper-containing enzyme proteins, it has the role of antioxidants, and has oxidase activity, and has the ability to catalyze the oxidation of polyphenols and polyamine substrates. It is generally believed that ceruloplasmin is synthesized by the liver, part of which is excreted by the biliary tract, and the urine content is very small. The determination of aeruginin has certain significance for the diagnosis of certain liver, gallbladder, kidney and other diseases.
2. FTH1 – Ferritin heavy chain
The ferritin heavy chain is an iron oxidase encoded by the FTH1 gene in humans. Ferritin is the main intracellular iron storage protein in prokaryotes and eukaryotes. It consists of 24 subunits of the heavy and light ferritin chains. Changes in the composition of ferritin subunits may affect the rate of iron absorption and release in different tissues. The main function of ferritin is to store iron in a soluble and non-toxic state. Defects in ferritin are associated with several neurodegenerative diseases. This gene has multiple pseudogenes. Several alternative splicing transcript variants have been observed, but their biological effectiveness has not been determined.
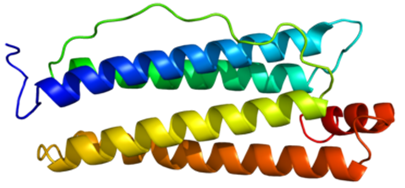 Figure 1. Structure of the FTH1 protein.
Figure 1. Structure of the FTH1 protein.
3. FTMT – Ferritin, mitochondrial
Mitochondria ferritin is a human iron peroxidase, encoded by the FTMT gene, and is classified as a metal-binding protein located in the mitochondria. After proteins are absorbed by mitochondria, they can be processed into mature proteins and assembled into functional ferritin shells.
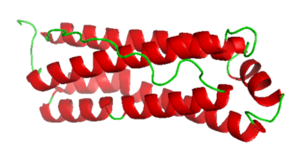 Figure 2. Mitochondrial Ferritin.
Figure 2. Mitochondrial Ferritin.
4. HEPH – Hephaestin
Heparin is involved in the metabolism and homeostasis of iron and copper. It is a transmembrane copper-dependent iron oxidase that is responsible for transporting dietary iron from intestinal intestinal epithelial cells to the circulatory system. The highest expression of heparin was found in the small intestine. It is confined to villous intestinal epithelial cells (where iron absorption occurs) and is almost absent from crypt cells. Hephaestin works in concert with ferroportin 1 to convert iron (II) Fe2+ to iron (III) Fe3+ and mediate iron efflux. Low levels of heparin are detected in the colon, spleen, and kidneys, as well as breast, placental, and trabecular bone cells, but their role in these tissues remains to be determined. Hephaestin is homologous to ceruloplasmin, which is a serum dehydrogenase protein involved in copper detoxification and storage.
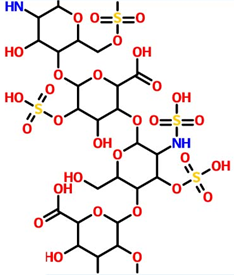 Figure 3. Structure of Heparin.
Figure 3. Structure of Heparin.
The regulation of heparin protein expression and the role of proteins in the context of iron metabolism and homeostasis are still active areas of research. Some studies have proposed mechanisms to control intestinal iron transport locally and systemically. In this mechanism, high dietary iron intake and sufficient iron storage can lead to downregulation of DMT1, iron transporter (Ireg1), and heparin proteins, thereby reducing the absorption of iron from the intestinal cells into the circulation is minimized. On the contrary, it is suggested that the state of low dietary intake and low iron storage can induce up-regulation of DMT1 as well as Ireg1 and heparin.
References
- Takahashi N.; et al. Single-chain structure of human ceruloplasmin: the complete amino acid sequence of the whole molecule. Proceedings of the National Academy of Sciences of the United States of America. 1984, 81 (2): 390-4.
- Ishikawa K.; et al. Prediction of the coding sequences of unidentified human genes. X. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res. 1988, 5 (3): 169-76.