Fungal ferroxidases (Fet3-type)
Ferroxidases are common in the biological world, including a series of reductases (such as nucleotide reductase, nitrate reductase, nitrite reductase, etc.), hydrogenase, nitrogenase, peroxidase (including Cytochrome peroxidase, myeloperoxidase, etc.), oxidase (including cytochrome c, oxidase, etc.), hydratase, etc. Most of these are heme enzymes, and there are many important iron-containing enzymes that are not Heme enzymes, iron-containing enzymes are involved in the electron transfer process. Iron is the trace element that the human body needs the most. Most of it exists in red blood cells in the form of hemoglobin. It is one of the body muscles and iron enzymes. The physiological effects of iron are: constituting heme, preventing anemia; participating in cytochrome synthesis, regulating tissue breathing and energy metabolism; maintaining the body's immunity and anti-infective ability. Hemoglobin is the main component of red blood cells and is responsible for carrying oxygen throughout the body for metabolism. If the iron supply is insufficient, the synthesis of hemoglobin will be affected and anemia will be caused. Medically called nutritional iron deficiency anemia, it is a common disease in children. For infants, due to the low iron content in breast milk, the iron obtained from the mother during the fetal period and stored in the body will be consumed in about 6 months after birth. If the supplementary food is not added in time, the baby will start to occur about 6 months after birth. Iron deficiency causes anemia symptoms.
Cytochrome c
Cytochrome complex or cyt c is a small hemoglobin that is associated with loose inner membrane of mitochondria. It belongs to the cytochrome c family of proteins and plays a major role in apoptosis. Unlike other cytochromes, cytochrome c is highly water-soluble and is an essential component of the electron transport chain, which carries an electron. When its iron atom is switched between ferrous and iron forms, it is able to undergo oxidation and reduction but does not combine with oxygen. It transfers electrons between complex III (Coenzyme Q-Cyt C reductase) and IV (Cyt C oxidase). In humans, cytochrome c is encoded by the CYCS gene.
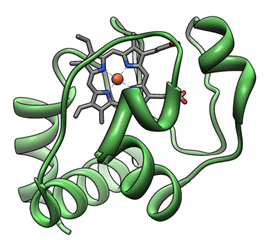 Figure 1. Three-dimensional structure of cytochrome c (green).
Figure 1. Three-dimensional structure of cytochrome c (green).
Oxidases
Oxidases are the main enzymes in peroxidases. Oxidases account for about half of the total peroxidases, including: urate oxidase, D-amino acid oxidase, L-amino acid oxidase and L -α-hydroxyacid oxidase and so on.
Cytochrome P450
Cytochrome P450 (CYPs) is a superfamily of enzymes containing heme as a cofactor, which functions as a monooxygenase. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, which are important for scavenging various compounds as well as hormone synthesis and breakdown. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids and hormones. CYP enzymes have been found in all kingdoms of life: animals, plants, fungi, protozoa, bacteria, archaea, and even viruses. However, they do not exist. For example, they have not been found in E. coli. There are more than 50,000 different CYP proteins known. Generally, CYP is a terminal oxidase in the electron transfer chain and is widely classified as a P450-containing system. The term "P450" is derived from a spectrophotometric peak at the maximum absorption wavelength (450 nm) of an enzyme in a reduced state and complexed with carbon monoxide. Most CYPs require a protein chaperone to pass one or more electrons to reduce iron (eventually to molecular oxygen).
Reference
- Gonzalez FJ.; et al. Human cytochromes P450: evolution and cDNA-directed expression. Environmental Health Perspectives. 1992, 98: 81-5.