Fungal pigment MCOs
Genomic analysis revealed multiple genes of blue polycopper oxidase (MCO) in various basidiomycetes. The entire genome is now available from saprophytic, white and brown rot species, animal and plant pathogens and mycorrhizal fungi. The total number of mco genes (from 1 to 17) and types differed among the species analyzed, and it was not easy to identify the link between gene distribution and fungal lifestyle. The type of mco gene may be present in one fungus and not present in another. In different organisms, different types of genes are reproduced during species formation. Phylogenetic analysis defines different laccase subfamilies (specific to Agaricus blazei), the classic Fe2 ++ oxidized Fet3-like iron oxidase, a potential iron oxidase/laccase that shows one or both of these enzyme functions, and pigment Different subfamilies of MCO aggregated enzymes and putative ascorbate oxidase. Laccases in the strict sense are laccases in the strict sense due to their proposed role in the degradation of wood, straw and plant litter, and the strong interest in these enzymes in biotechnology. However, the biological functions of laccase and other MCOs are rarely mentioned. The functions of substrate degradation, symbiosis and pathogen interaction, development, pigmentation and copper homeostasis are proposed. In most cases, evidence of biological function is fairly indirect due to the relevance of expression. A variety of factors hinder the study of biological functions, such as the difficulty in defining a suitable biological system for molecular research, the substrate spectrum of multi-copper oxidases usually has a wide overlapping overlap, insufficient understanding of natural substrates, low or multiple expression difficult enzymes, and the difficulty of heterologously expressing enzymes.
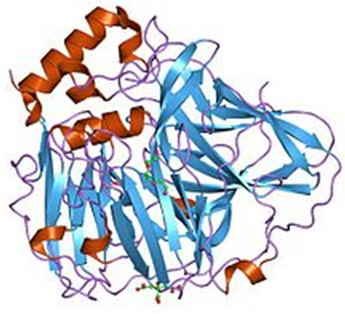 Figure 1. crystal structures of e. coli laccase cueo under different copper binding situations.
Figure 1. crystal structures of e. coli laccase cueo under different copper binding situations.
1. Ceruloplasmin
Ceruloplasmin is a copper-containing α2 glycoprotein with a molecular weight of about 120,000 to 160,000, which is not easy to purify. It is a single-chain polypeptide containing 6-7 copper atoms per molecule. It is blue due to copper and contains about 10% sugar. The terminal sialic acid is linked to the polypeptide chain and has genetic polymorphism. Its role is to regulate the distribution of copper in various parts of the body, to synthesize copper-containing enzyme proteins, it has the role of antioxidants, and has oxidase activity, and has the ability to catalyze the oxidation of polyphenols and polyamine substrates. It is generally believed that ceruloplasmin is synthesized by the liver, part of which is excreted by the biliary tract, and the urine content is very small. The determination of aeruginin has certain significance for the diagnosis of certain liver, gallbladder, kidney and other diseases.
2. Laccase
Laccase is a polyphenol oxidase containing four copper ions. It belongs to the copper blue oxidase and exists as a monomer glycoprotein. Laccase exists in mushrooms, bacteria and plants, and can also survive in the air. The only product after the reaction is water, so it is essentially an environmentally friendly enzyme. The unique catalytic properties of laccase make it widely used in biological detection, and as an efficient biodetector, it becomes an effective tool and means for the analysis of components such as substrates, coenzymes and inhibitors. As environmental protection awareness has been gradually valued in recent years, laccase has become the research object of many scholars in recent years. Laccase is a copper protein, blue, with a molecular weight of about 120,000 and containing 4 atomic copper, which can be inhibited by CN-. Laccase can oxidize polyphenols, promote the methoxy substitution of phenol and diamine, and oxidize almost all substrates with similar structure of p-polyphenol. In addition, some fungal laccases can also oxidize monophenols such as cresol and ascorbic acid.
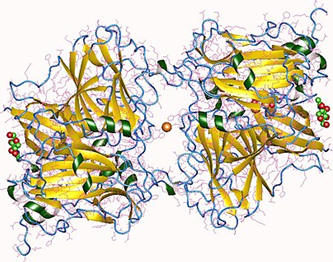 Figure 2. L-ascorbate oxidase.
Figure 2. L-ascorbate oxidase.
3. Ascorbate oxidase
Ascorbate oxidase is a copper-containing enzyme that is located in the cytoplasm or combined with the cell wall and is coupled with other redox reactions to play the role of terminal oxidase. It can catalyze the oxidation of ascorbic acid and has anti-aging effects. Has an important role in the metabolism of substances. Under the catalysis of this enzyme, molecular oxygen can oxidize ascorbic acid to dehydroascorbic acid, which exists in the cytosol and cell wall, and the enzyme contains copper.
4. Nitrite reductase
Nitrite reductases (NiRs) are a class of enzymes that catalyze the reduction of nitrite. Most of the nitrite reductases are intracellular enzymes, which can effectively degrade nitrite in the cell. This enzyme is an oxidoreductase. The catalytic reaction process requires the participation of electron donors and transferors, and the reaction needs Under oxygen conditions. Nitrite reductase is widely present in microorganisms and plants and is a key enzyme in the nitrogen cycle of nature. It can degrade nitrite to NO or NH3, thereby reducing the accumulation of nitrite nitrogen in the environment and reducing the accumulation of nitrite resulting toxic effects on organisms.
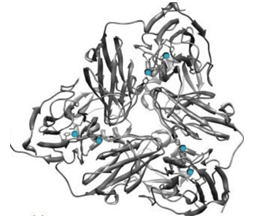 Figure 3. Protein structure of nitrite reductases.
Figure 3. Protein structure of nitrite reductases.
Reference
- Bento I.; et al. Dioxygen reduction by multi-copper oxidases; a structural perspective. Dalton Transactions. 2005, (21): 3507-13.