Signal Transduction
The transmitting of a chemical or physical signal through a cell as a series of molecular events is called signal transduction. The most common signal transduction is protein phosphorylation catalyzed by protein kinases, and ultimately results in a cellular response. Generally, proteins are named as receptors that function as signal transducers in the procedure of signal transduction, mainly responsible for the detection of stimuli, while in some cases the term sensor is adopted.
In eukaryotic cells, most intracellular proteins activated by a ligand/receptor interaction are endowed with enzymatic activity, such as tyrosine kinase and phosphatases. This kind of enzymes is covalently bound to the receptor, some of which could further produce second messengers such as cyclic AMP and inositol trisphosphate that is responsible for the release of intracellularly stored calcium into the cytoplasm. Other activated proteins could interact with adaptor proteins that promote the interaction of signaling proteins and the coordination of signaling complexes necessary to respond to a particular stimulus. Both enzymes and adaptor proteins are responsive to a variety of second messenger molecules, because many activated enzymes as part of signal transduction contain specialized protein domains that could bind to specific secondary messenger molecules. For example, the binding of calcium ions with the helix-loop-helix structural domains of calmodulin allows it to interact with calmodulin-dependent kinase and activate it.
Tyrosine, Ser/Thr and Histidine-specific Protein Kinases as Extracellular Receptors
Receptor tyrosine kinases (RTKs) are the high-affinity transmembrane proteins receptors for many polypeptide growth factors, cytokines, and hormones. They possess an intracellular kinase domain and an extracellular domain that could bind with ligands, such as growth factor receptors and the insulin receptor. Many Ser/Thr and dual-specificity protein kinases important for signal transduction could either act upon the downstream of RTKs or emerge as membrane-embedded or cell-soluble versions in their own right. About 560 known protein kinases and pseudokinases that are encoded by the human kinome are involved in the process of signal transduction. Although histidine-specific protein kinases prevalently found in prokaryotes, fungi, and plants are structurally distinct from other protein kinases, they also take effects in a two-component signal transduction route. Wherein a phosphate group from ATP is first attached to a histidine residue within the kinase, and the next transfer of the group to an aspartate residue on a receptor domain of a different protein or the kinase itself activate the aspartate residue.
Acting Mechanism
RTKs need to form dimers in the plasma membrane to conduct signal transduction, and the dimer could be stabilized through the binding of ligands with the receptor. Within the intracellular kinase domains of the RTKs, the interaction between cytoplasmic domains provokes the autophosphorylation of tyrosine residues, and thus leads to conformational changes. Herein, the kinase domains of receptors are subsequently activated, which induces the phosphorylation signaling cascades of downstream cytoplasmic molecules that accelerate various cellular processes including cell differentiation and metabolism. Similar to G protein–coupled receptors (GPCRs), proteins that bind to guanosine-5'-triphosphate play a major role in signal transduction from the activated RTK to the cell. Under the circumstance, the G proteins are members of the Ras, Rho, and Raf families, and are collectively referred to as small G proteins. They usually serve as molecular switches by being tethered to membranes though the isoprenyl groups linked to their carboxyl ends. Once activated, they assign proteins to specific membrane subdomains where they execute signal conduction. Activated RTKs in turn arouse small G proteins activating guanine nucleotide exchange factors, which can stimulate more small G proteins after activation, thus amplifying the original signal of receptor. The mutation of certain RTK genes could cause the expression of receptors that are present at a constitutively activated state, which may be an inducement of tumor.
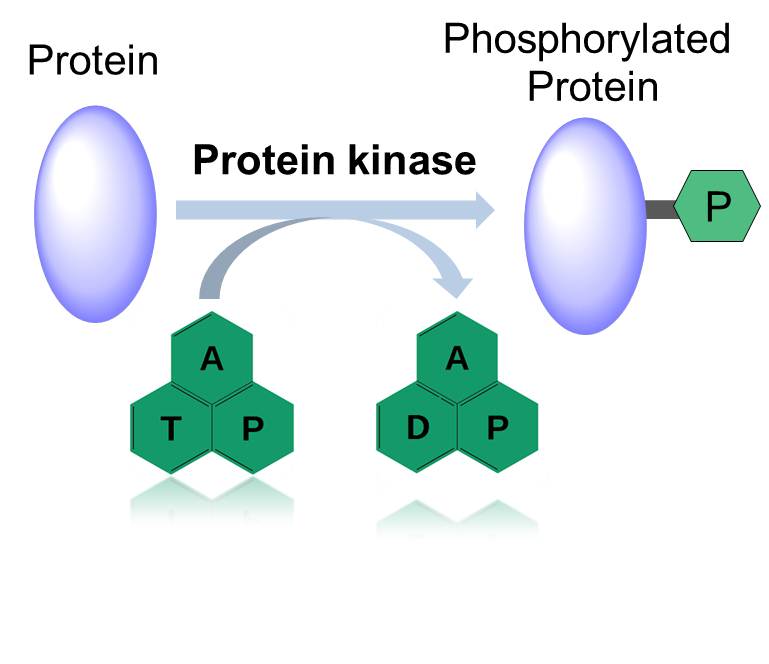 Figure 1. Action Mode of Protein Kinase.
Figure 1. Action Mode of Protein Kinase.
Characteristics
RTK signal transduction pathway shares common characteristic of that the receptor itself has tyrosine protein kinase (TPK) activity, and the ligand is mainly growth factor. The RTK pathway is closely related to cell proliferation, cell hypertrophy and tumor formation. After the ligand binds to the extracellular domain of receptor, the dimerization of the receptor endows itself TPK activity and catalyzes the autophosphorylation of tyrosine residues in the intracellular domain. The downstream signal transduction of RTK is activated by a series of serine/threonine protein kinases: (1) activation of mitogen-activated protein kinase (MAPK), (2) activation of protein kinase C (PKC), and (3) activation of phosphatidylinositol-3 kinase (PI3K), thus triggering corresponding biological effects.
Other Roles in Signal Transduction
A variety of intracellular protein kinases also participate in the integrin-mediated signal transduction, and integrin-linked kinase works as the main coordinator. In an experimental model plant Arabidopsis thaliana, one of the integrin-linked kinase genes, ILK1, has been indicated to be a critical element in both plant immune response to signal molecules from bacteria and plant sensitivity to salt and osmotic stress.
After activation, capase proteases as the executors of apoptosis could further cleave substrates, such as poly ADP-ribose polymerase (PARP) that is associated with DNA repair and gene integrity monitoring. When PARP is cleaved, the loss of normal function leads to an enhancement on the activity of nucleic acid endonuclease inhibited by PARP and cleaves DNA between nucleosomes, eventually inducing apoptosis. This process can be summarized as follows: death receptor contains death domain cytoplasmic proteins - capase protease family - substrate PARP - chromosome breakage - apoptosis.
Reference
- Sako Y, Minoghchi S, Yanagida T. Single-molecule imaging of EGFR signalling on the surface of living cells. Nat Cell Biol, 2000, 2(3):168–172.