-
Enzymology Assays
- Fluorometric Enzyme Assays
- Chemiluminescent Enzyme Assays
- Light Scattering Enzyme Assays
- Microscale Thermophoresis Enzyme Assays
- Radiometric Enzyme Assays
- Calorimetric Enzymes Assays
- Enzyme Catalytic Mechanisms
- Development of Analysis Methods
- Enzyme Kinetics
-
Enzyme Activity Measurement
-
Enzyme Activity Measurement for Phosphorus Transferases
- Enzyme Activity Measurement for Glucokinase
- Enzyme Activity Measurement for Galactokinase
- Enzyme Activity Measurement of Ribokinase Using Chromatographic Assays
- Enzyme Activity Measurement for Thymidine Kinase
- Enzyme Activity Measurement for Glycerol Kinase
- Enzyme Activity Measurement for Pyruvate Kinase
- Enzyme Activity Measurement for Fucokinase
- Enzyme Activity Measurement of Polynucleotide 5'-Hydroxyl-kinase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Diphosphate-Fructose-6-Phosphate 1-Phosphotransferase
- Enzyme Activity Measurement for Diacylglycerol Kinase (ATP)
- Enzyme Activity Measurement for ADP-Specific Glucokinase
- Enzyme Activity Measurement of N-Acetylhexosamine 1-Kinase
- Enzyme Activity Measurement of Acetate Kinase
- Enzyme Activity Measurement of Polyphosphate Kinase
- Enzyme Activity Measurement of Adenylate Kinase
- Enzyme Activity Measurement of dTMP Kinase
- Enzyme Activity Measurement of (d)CMP Kinase
- Enzyme Activity Measurement of Phosphorylase Kinase
- Enzyme Activity Measurement for Phosphoglycerate Kinase
- Enzyme Activity Measurement of DNA Nucleotidylexotransferase
- Enzyme Activity Measurement of UTP-Monosaccharide-1-Phosphate Uridylyltransferase
- Enzyme Activity Measurement of NAD+ Kinase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Creatine Kinase
- Enzyme Activity Measurement for Nucleoside-Diphosphate Kinase
- Enzyme Activity Measurement for Guanylate Kinase
- Enzyme Activity Measurement for Pantetheine-Phosphate Adenylyltransferase
- Enzyme Activity Measurement for Sulfate Adenylyltransferase
- Enzyme Activity Measurement for UDP-Glucose-Hexose-1-Phosphate Uridylyltransferase
- Enzyme Activity Measurement for Mannose-1-Phosphate Guanylyltransferase
- Enzyme Activity Measurement for N-Acylneuraminate Cytidylyltransferase
- Enzyme Activity Measurement for RNA-Directed DNA Polymerase
- Enzyme Activity Measurement for Non-Specific Serine/Threonine Protein Kinase
-
Enzyme Activity Measurement for Glycosyl, Hexosyl, and Pentosyl Transferases
- Enzyme Activity Measurement for 1,4-Alpha-Glucan Branching Enzyme Using Spectrophotometric Assays
- Enzyme Activity Measurement for Beta-N-Acetylglucosaminyl-Glycopeptide Beta-1,4-Galactosyltransferase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Ganglioside Galactosyltransferase Using Chromatographic Assays
- Enzyme Activity Measurement for 3-Galactosyl-N-Acetylglucosaminide 4-Alpha-L-Fucosyltransferase Using Chromatographic Assays
- Enzyme Activity Measurement for N-Acetyllactosaminide Beta-1,6-N-Acetylglucosaminyl Transferase Using Chromatographic Assays
- Enzyme Activity Measurement for Hyaluronan Synthase Using Chromatographic Assays
- Enzyme Activity Measurement for Lactosylceramide 4-Alpha-Galactosyltransferase Using Chromatographic Assays
- Enzyme Activity Measurement for Purine-Nucleoside Phosphorylase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Uridine Phosphorylase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Thymidine Phosphorylase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Nucleoside Deoxyribosyltransferase Using Chromatographic Assays
- Enzyme Activity Measurement of Adenine Phosphoribosyltransferase Using Chromatographic Assays
- Enzyme Activity Measurement of Hypoxanthine Phosphoribosyltransferase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Beta-Galactoside Alpha-2,3-Sialyltransferase
- Enzyme Activity Measurement of Glycoprotein 3-Alpha-L-Fucosyltransferase Using Spectrophotometric Assays
- Enzyme Activity Measurement of N-Acetyllactosamine Synthase
- Enzyme Activity Measurement of N-Acetyllactosaminide 3-Alpha-Galactosyltransferase
- Enzyme Activity Measurement of Polypeptide N-Acetylgalactosaminyltransferase
- Enzyme Activity Measurement of Glycoprotein-Fucosylgalactoside Alpha-N-acetylgalactosaminyltransferase
- Enzyme Activity Measurement of Maltose Phosphorylase
- Enzyme Activity Measurement of Sucrose Phosphorylase
- Enzyme Activity Measurement for Glycogen Phosphorylase
- Enzyme Activity Measurement for Dextransucrase
- Enzyme Activity Measurement for Levansucrase
-
Enzyme Activity Measurement for Hydrolases Acting on Ester Bonds
- Measurement for Carboxylic Ester Hydrolases Acting on Ester Bonds
- Enzyme Activity Measurement for Phosphoric Monoester Hydrolases Acting on Ester Bonds
- Enzyme Activity Measurement for Phosphoric Diester Hydrolases
- Enzyme Activity Measurement for Sulfuric Ester Hydrolases Acting on Ester Bonds
- Enzyme Activity Measurement for Phosphoric Triester Hydrolases Acting on Ester Bonds
- Enzyme Activity Measurement for EC 3.1.15 and EC 3.1.16 Acting on Ester Bonds
- Enzyme Activity Measurement for EC 3.1.21 and EC 3.1.22 Acting on Ester Bonds
- Enzyme Activity Measurement for EC 3.1.26 and EC 3.1.27 Acting on Ester Bonds
- Enzyme Activity Measurement for EC 3.1.30 and EC 3.1.31 Acting on Ester Bonds
- Enzyme Activity Measurement for Oxidoreductases Interacting with Inorganics
- Enzyme Activity Measurement for Glycosylases
- Enzyme Activity Measurement for Isomerases
- Kinase Screening and Profiling Services
-
Enzyme Activity Measurement for Oxidoreductases Acting on Carbon Using Spectrophotometric Assays
- Enzyme Activity Measurement for Oxidoreductases Acting on CH-OH Group with Oxygen as Acceptor
- Enzyme Activity Measurement for Oxidoreductases Acting on CH-OH Group with Quinone or Similar Compound as Acceptor
- Enzyme Activity Measurement for Oxidoreductases Acting on CH-OH Group with Other Acceptors
- Enzyme Activity Measurement for Oxidoreductases Acting on Aldehyde or Oxo Group with Iron-Sulfur Protein as Acceptor
- Enzyme Activity Measurement for Oxidoreductases Acting on Aldehyde or Oxo Group With Other Acceptors
- Enzyme Activity Measurement for Oxidoreductases Acting on CH-CH Group With NAD or NADP as Acceptor
- Enzyme Activity Measurement for Oxidoreductases Acting on CH or CH2 Groups With NAD or NADP as Acceptor
- Enzyme Activity Measurement for Oxidoreductases Acting on CH or CH2 Groups With Oxygen as Acceptor
- Enzyme Activity Measurement for Oxidoreductases Acting on CH or CH2 Groups With Other Acceptors
- Enzyme Activity Measurement for Oxidoreductases Acting on CH-CH Group With Oxygen
- Enzyme Activity Measurement for Oxidoreductases Acting on Aldehyde or Oxo Group With NAD or NADP as Acceptor
- Enzyme Activity Measurement for Oxidoreductases Acting on CH-OH Group with Cytochrome as Acceptor
-
Enzyme Activity Measurement for Oxidoreductases Acting on CH-OH Group with NAD or NADP as Acceptor
- Enzyme Activity Measurement for Alcohol Dehydrogenases Using Spectrophotometric Assays
- Enzyme Activity Measurement for Alcohol Dehydrogenase (NADP+) Using Spectrophotometric Assays
- Enzyme Activity Measurement of (R, R)-Butanediol Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Glycerol Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Pyridoxine 4-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Testosterone 17Beta-Dehydrogenase (NADP+) Using Spectrophotometric Assays
- Enzyme Activity Measurement for 3-Hydroxypropionate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Tagaturonate Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Testosterone 17Beta-Dehydrogenase(NAD+) Using Spectrophotometric Assays
- Enzyme Activity Measurement of Propanediol-Phosphate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Estradiol 17Beta-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for 4-Hydroxybutyrate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Glycerol-3-Phosphate Dehydrogenase (NAD+) Using Spectrophotometric Assays
- Enzyme Activity Measurement of D-Xylulose Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement for 2-Hydroxy-3-Oxopropionate Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement for L-Xylulose Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Phosphogluconate 2-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for D-Arabinitol 4-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for L-Arabinitol 4-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Isocitrate Dehydrogenase (NADP+) Using Spectrophotometric Assays
- Enzyme Activity Measurement for L-Arabinitol 2-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Isocitrate Dehydrogenase (NAD+) Using Spectrophotometric Assays
- Enzyme Activity Measurement for L-Iditol 2-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Malate Dehydrogenase (Oxaloacetate-Decarboxylating) (NADP+) Using Spectrophotometric Assays
- Enzyme Activity Measurement for D-iditol 2-dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Malate Dehydrogenase (Decarboxylating) Using Spectrophotometric Assays
- Enzyme Activity Measurement for Galactitol 2-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Malate Dehydrogenase (Oxaloacetate-Decarboxylating) Using Spectrophotometric Assays
- Enzyme Activity Measurement for Mannitol-1-Phosphate 5-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Inositol 2-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for 17 Beta Hydroxysteroid Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Aldehyde Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Glucuronolactone Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Glyoxylate Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement of L-Lactate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of D-Lactate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Glycerate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Shikimate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of 3-Hydroxybutyrate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Mevaldate Reductase (NADPH) Using Spectrophotometric Assays
- Enzyme Activity Measurement of Hydroxymethylglutaryl-CoA Reductase (NADPH) Using Spectrophotometric Assays
- Enzyme Activity Measurement of 3-Hydroxyacyl-CoA Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Allyl-Alcohol Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Lactaldehyde Reductase (NADPH) Using Spectrophotometric Assays
- Enzyme Activity Measurement for Fructuronate Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Acetoacetyl-CoA Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Malate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Phosphogluconate Dehydrogenase (NADP+-Dependent, Decarboxylating) Using Spectrophotometric Assays
- Enzyme Activity Measurement of L-Gulonate 3-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of L-Arabinose 1-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Glucose 1-Dehydrogenase [NAD(P)+] Using Spectrophotometric Assays
- Enzyme Activity Measurement of D-Galactose 1-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Glucose-6-Phosphate Dehydrogenase (NADP+) Using Spectrophotometric Assays
- Enzyme Activity Measurement for 3Alpha-Hydroxysteroid 3-Dehydrogenase (Si-specific) Using Spectrophotometric Assays
- Enzyme Activity Measurement for Mannitol 2-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement for Gluconate 5-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Uronate Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of IMP Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of N-Acylmannosamine 1-Dehydrogenase Using Spectrophotometric Assays
- Enzyme Activity Measurement of Succinic Semialdehyde Reductase Using Spectrophotometric Assays
- Enzyme Activity Measurement for 12 Alpha-hydroxysteroid Dehydrogenase
- Enzyme Activity Measurement for D-Malate Dehydrogenase (Decarboxylating) Using Spectrophotometric Assays
- Enzyme Activity Measurement for Ketol-Acid Reductoisomerase (NADP+)
- Enzyme Activity Measurement for Sorbitol-6-Phosphate 2-Dehydrogenase
-
Enzyme Activity Measurement for Phosphorus Transferases
- Enzyme Discovery
- Experiment Consulting and Design Service
-
Screening of Substrates, Inhibitors, and Other Ligands
- Best Substrate for An Enzymatic Reaction
- Natural Substrates of An Enzyme
- Virtual Screening of Enzyme Inhibitors
- High-throughput Screening of Inhibitors
- Structure-based Inhibitor Design
- Ligand-based Inhibitor Design
- Enzyme Screening Against Substrates
- Identification of a Target Enzyme
- Screening of Enzyme Activators
- Evaluation of Cofactors and Additives
- Enzyme Aggregation and Oligomerization
- Enzyme Folding Facilitators
-
Enzyme Engineering and Modification
- Abzymes
- Synzymes
- Enzyme Labelling Services
- Enzyme Engineering by Directed Evolution
- Enzyme Engineering by Rational Design
- Enzyme Covalent Chemical Modifications
- Enzyme Engineering by De Novo Design
- Enzyme Engineering by Site-directed Mutagenesis
- Enzyme Engineering by Random Mutagenesis and DNA Shuffling
- Phage Display and mRNA Display for Enzyme Engineering
- Enzyme Modification by Immobilization
- Enzyme Encapsulation
- Incorporation of Unnatural Amino Acids
- Enzyme Conjugation
-
Biocatalysis Services
- Multi-Enzyme Cascade Reaction Systems
- Whole Cell Biocatalysts
- Biocatalyst Immobilization and Modification
- Computational Modeling
- Continuous Directed Evolution
- Rational Design
- Substrate Profiling
- Catalytic Assays
- Mechanism Modeling and Investigation
- Selection and Modification of Cofactors
- Codon Optimization
- Genome Engineering
- Metabolic Flux Analysis
- Natural Biocatalyst Sampling and Screening
- Pathway Engineering
- Process Optimization
- Process Scale-Up
- Metabolic Pathway Engineering
-
Enzyme Expression and Purification
-
Enzyme Expression and Production
- Expression Evaluation and Optimization
- E. coli Enzyme Expression System
- Enzyme Expression and Production in Other Bacteria
- Express Enzymes in Fungi
- Express Enzymes in Baculovirus/Insect Cells
- Transgenic Plants Enzyme Expression System
- Enzyme Expression in Transgenic Animals
- Enzyme Expression in Methylotrophs
- Enzymes Expression in Other Yeasts
-
Enzyme Purification
- Enzyme Quality Certification
- Enzyme Purification by Affinity Column for Tagged Enzymes
- Enzyme Purification by Immunoprecipitation
- Enzyme Characterization Service
- Enzyme Purification by Ion Exchange Chromatography
- Enzyme Purification by Size Exclusion (SEC) and Gel Filtration (GF)
- Endotoxin Removal in Enzyme Purification
- Enzyme Purification by Hydrophobic Interaction Chromatography
- Enzyme Purification by Electrophoresis
- Solubility-Based Enzyme Purification
- Solubilizing Inclusion Body
- Enzyme Recovery and Refolding
-
Enzyme Expression and Production
-
Enzyme Stabilization and Formulation
- Enzyme Stabilization by Immobilization
- Enzyme Stabilization by Micelle and Reverse Micelle
- Chemical Modifications of Enzymes
- Enzyme Stabilization by Engineering
- Ionic Liquid and Polymer Coating for Enzyme Stabilization
- Enzyme Stabilization by Use of Additives
- Stabilization in Organic Solvents
- Real-time and Accelerated Stability Test
- Enzyme pH Stability
- Enzyme Thermal Stability
- Ionic Strength on Enzyme Stability
- Enzyme Formulation
- Industrial Enzyme Production
-
Nanozyme Services and Applications
- Nanozyme-Based Disease Diagnosis and Treatment
- Bacterial Detection and Antibacterial Applications Based on Nanozymes
- Nanozyme-Based Food Safety Monitoring
- Nanozyme-Based Catalytic Degradation of Pollutants
- Nanozyme-Based Wastewater Treatment
- Nanozyme-Based Biosensors
- Nanozyme-Based UV-Blocking Sunscreen
-
Design and Construction Services for Nanozymes
- Construction of Cerium-Based Nanozymes
- Construction of Ferrum-Based Nanozymes
- Construction of Copper-Based Nanozymes
- Construction of Manganese-Based Nanozymes
- Construction of Molybdenum-Based Nanozymes
- Construction of Cobalt-Based Nanozymes
- Construction of Platinum-Based Nanozymes
- Construction of Platinum-Based Nanozymes
- Construction of Gold-Based Nanozymes
- Construction of Iridium-Based Nanozymes
- Construction of Ruthenium-Based Nanozymes
- Construction of Non-Metallic Nanozymes
- Nanozymes-Based Detection Services
Our Products Cannot Be Used As Medicines Directly For Personal Use.


Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Enzyme Activity Measurement for Peroxidase
Creative Enzymes is the leading company in the field of enzyme activity assays, which is well known to provide the best customer satisfaction. We have a group of brilliant scientists who have been working on enzyme activity assays for many years. Here we are proud to offer the catalytic activity assay for peroxidase. The unique advantage of our spectrophotometric analysis allows us to surpass our competitors and ensures the reliability of the test results.
Peroxidases (EC 1.11.1.7) are a large family of ubiquitous oxidative heme-containing enzymes which can be isolated from plants, bacteria, and fungi, as well as mammalian sources. The peroxidases belong to a family of glycoproteins containing iron atoms as a prosthetic group and different quantities of carbohydrate residues. Peroxidases are involved in multiple biological processes which include cell wall synthesis and degradation, stress response, signaling during oxidative stress, and removal of xenobiotics.
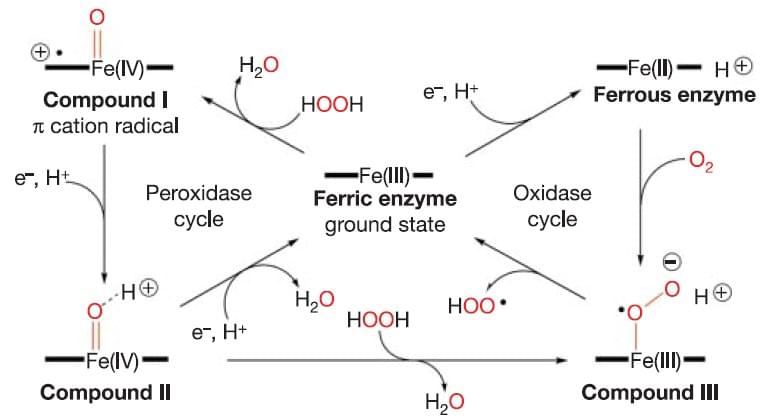 Figure 1: Reactions catalyzed by peroxidase.
Figure 1: Reactions catalyzed by peroxidase.
Reference: Berglund G I et al. Nature, 2002, 417(6887): 463-468.
Heme-containing redox enzymes join in a strikingly diverse range of chemistry, yet all biological oxidation reactions catalyzed by these enzymes involve very similar high-oxidation-state intermediates whose reactivity is modulated by the protein environment. The main overall reaction catalyzed by peroxidases can be summarized as H2O2 + 2RH → 2H2O + 2R (where RH stands for a reducing substrate and R is a free radical product). This reaction involves several steps with two highly oxidized heme intermediates, compound I and compound II (Figure 1). Additionally, peroxidases can also convert hydrogen peroxide to water and dioxygen (2H2O2 → 2H2O + O2). This is the catalase-like activity of the enzyme, but in contrast to true catalases, the rate of this reaction in peroxidases is slow. Peroxidases activity can be influenced by small molecule ligands, including carbon monoxide, cyanide, azide, and fluoride, formate and acetate. Both acetate and formate bind to ferric horseradish peroxidase, and formate binds more strongly than acetate.
Additionally, peroxidases also play an important role in the environment, agriculture and manufacturing industries. For example, peroxidases can be used for the treatment of industrial waste waters; peroxidases can be used in many manufacturing processes (e.g., as adhesives for linings of drums, cans and car parts). Moreover, peroxidases could be used to polymerize anilines and phenols in organic solvent matrices. Therefore, more detailed information about peroxidases should be obtained due to its significant value. However, the enzymatic activity assay of this enzyme has not been well established probably because of its complicated chemistry behind the enzymatic reaction and its activity regulation by small molecule ligands. Fortunately, Creative Enzymes, equipped with the leading technique, the up-to-date experiment instruments, and the experienced technical experts, promises our customers to supply the most precise test results for the activity quantification of peroxidases. Creative Enzymes is always looking forward to collaborating with you!
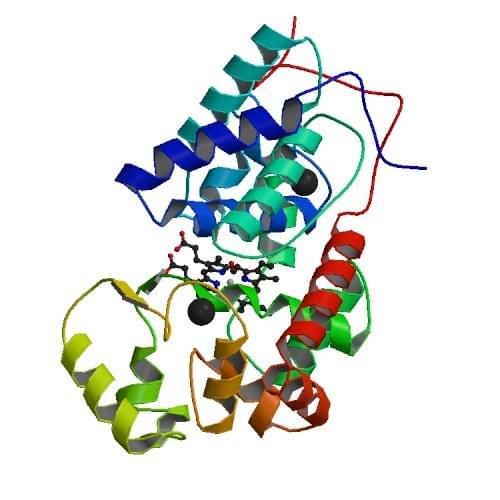 Figure 2: The crystal structure of ferric horseradish peroxidase C1A from Armoracia rusticana.
Figure 2: The crystal structure of ferric horseradish peroxidase C1A from Armoracia rusticana.
PDB: 1W4W
| Catalog | Product Name | EC No. | CAS No. | Source | Price |
|---|---|---|---|---|---|
| CEFX-029 | Myeloperoxidase-Agarose | EC 1.11.1.7 | Leukocytes in s... | Inquiry | |
| CEFX-027 | Human Sputum Myeloperoxidase, Gamma Irradiated | EC 1.11.1.7 | Leukocytes in s... | Inquiry | |
| CEFX-026 | Human Sputum Leucocyte Myeloperoxidase | EC 1.11.1.7 | Leukocytes in s... | Inquiry | |
| EXWM-0509 | peroxidase | EC 1.11.1.7 | 9003-99-0 | Inquiry | |
| NATE-0966 | Native Human Lactoperoxidase | EC 1.11.1.7 | 9003-99-0. | Human Saliva | Inquiry |
| NATE-0550 | Native Horseradish Peroxidase | EC 1.11.1.7 | Horseradish Roo... | Inquiry | |
| NATE-0461 | Native Human Myeloperoxidase Isoform C | EC 1.11.1.7 | 9003-99-0 | Human Neutrophi... | Inquiry |
| NATE-0460 | Native Human Myeloperoxidase Isoform B | EC 1.11.1.7 | 9003-99-0 | Human Neutrophi... | Inquiry |
| NATE-0458 | Native Human Myeloperoxidase Isoform A | EC 1.11.1.7 | 9003-99-0 | Human Neutrophi... | Inquiry |
| NATE-0228 | Native Human Eosinophil Peroxidase | EC 1.11.1.7 | 9003-99-0 | Human Eosinophi... | Inquiry |
| NATE-0549 | Native Bjerkandera adusta Peroxidase | EC 1.11.1.7 | 9003-99-0 | Bjerkandera adu... | Inquiry |
| NATE-0457 | Native Human Myeloperoxidase | EC 1.11.1.7 | 9003-99-0 | Human leuk ocyt... | Inquiry |
| NATE-0418 | Native Bovine Lactoperoxidase | EC 1.11.1.7 | 9003-99-0 | Bovine milk | Inquiry |
| PHAM-231 | Native Horseradish Peroxidase | EC 1.11.1.7 | 9003-99-0 | Horseradish | Inquiry |