
Introduction
Humans are continuously exposed to a wide variety of environmental chemicals, drugs, food additives, and pollutants that could eventually impair cellular metabolism with detrimental effects on health. Defense systems that contain as their centerpiece the unique hemeprotein cytochrome P450 (CYP) have evolved to protect organisms against toxic compounds. However, CYP-mediated biotransformation may result in metabolic activation of environmental chemicals to reactive carcinogenic products, a process often referred to as lethal synthesis.
CYPs continue to receive the attention of biochemists and pharmacologists because of their pivotal role in the detoxification of xenobiotics as well as diverse endogenous compounds such as steroids, bile acids, unsaturated fats, prostaglandins, and leukotrienes. CYPs play a predominant role in the metabolism of carcinogens and drugs that mitigate cancer growth. Thus, inhibitors of CYP enzymes may potentially serve as anticancer agents.
Occurrence and Functions
CYPs constitute a superfamily of hemeproteins ubiquitously found in animals, plants, fungi, and bacteria. In mammals, CYP enzymes are present in all tissues with highest concentrations in the liver and small intestine. These are membrane-bound proteins that abound in the microsomal fraction of the liver, and have a crucial role in bile acid biosynthesis, and metabolism of foreign compounds such as drugs, environmental pollutants, and carcinogens. CYPs are also present in the mitochondrial inner membranes of steroidogenic tissues such as adrenal cortex, testis, ovary, breast, and placenta, and are involved in the synthesis and degradation of endogenous steroid hormones. In addition, CYP enzymes play a major role in vitamin metabolism, oxidation of unsaturated fatty acids, and cholesterol biosynthesis. Specific functions have been documented for CYPs in the brain including regulation of endogenous GABAA receptor agonists, maintenance of brain cholesterol homeostasis and elimination of retinoids. Thus CYPs play a central role in cellular metabolism and maintain cellular homeostasis.
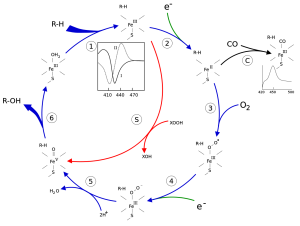
Figure 1. The P450 catalytic cycle.
Identification of moonlighting CYPs has added a new dimension to the complexity and the repertoire of functions catalysed by this large superfamily. CYP170A1, the first moonlighting CYP to be discovered, was found to function both as a monooxygenase and terpene synthase. This bifunctional protein isolated from the Gram-positive bacterium Streptomyces, was shown to switch between two distinct active sites in its structure in order to catalyse two unrelated biochemical activities. CYP17 (17α-hydroxylase/17–20 lyase), a microsomal enzyme that plays a role in steroid hormone biosynthesis, functions as a 17α-hydroxylase during the biosynthesis of cortisol in the adrenal cortex, whereas in the gonads it is involved in the biosynthesis of androgens as a 17–20 lyase. CYP7B1 performs a broad spectrum of functions in diverse tissues. This enzyme functions as a steroid 7α-hydroxylase in the brain using pregnenolone and dehydroepiandrosterone as substrates. In the liver, CYP7B1 catalyzes bile acid synthesis with both 25- and 27-hydroxycholesterol as substrates. CYP7B1 is involved in the inactivation of the 19-carbon steroid, 5α-androstane-3β, 17β-diol that binds to the estrogen receptor in the prostate gland. In addition, the enzyme also catalyzes the conversion of dehydroepiandrosterone to 7α-hydroxydehydroepiandrosterone that accumulates in synovial fluid in patients with rheumatoid arthritis.
Reference:
Palrasu M, Siddavaram N. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Current Drug Targets, 2018, 19(1).
Related Products at Creative Enzymes:

Leave a Reply