Bilirubin oxidases
Bilirubin is the product of normal catabolism of iron-containing porphyrin compounds in animals. Bilirubin plays an important role in the body. It is an antioxidant that helps the regeneration of liver cells. However, once metabolic abnormalities occur, bilirubin will accumulate in the body, forming hyperbilirubinemia, and even jaundice. Bilirubin oxidase can catalyze the reaction of bilirubin to produce non-toxic bilirubin. It is mainly used to determine the content of bilirubin in serum, and the amount of bilirubin in serum can be used as an important basis for diagnosis of hepatobiliary disease It can also provide information for pathological analysis. In recent years, bilirubin oxidase has also been used in dye degradation and bio-battery manufacturing, which has greatly expanded its application range.
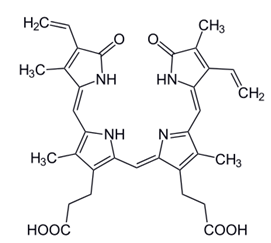 Figure 1. Structure of bilirubin.
Figure 1. Structure of bilirubin.
Source of bilirubin oxidase
Bilirubin oxidase is a polyphenol oxidase. The first research on bilirubin oxidase was by Murao and Tanaka, two foreign scholars. They purified bilirubin oxidase from a fungus of the genus Lactobacillus. It laid an important theoretical foundation for the subsequent research and application of bilirubin oxidase. The source of bilirubin oxidase is particularly extensive, and Lactobacillus is its main source and high-yielding bacteria. The microorganisms that produce bilirubin oxidase include Streptomyces, Frankia, Ganoderma lucidum, and Penicillium violaceum. Maitins found in research that B. subtilis spore coat protein also has bilirubin oxidase activity. In addition, foreign scientific research People have also isolated bilirubin oxidase in plants such as potatoes, onions, alfalfa and tomatoes.
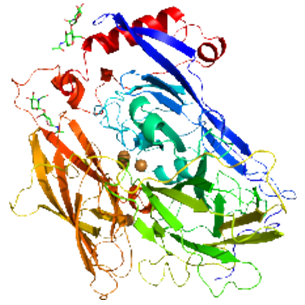 Figure 2. Protein structure of bilirubin oxidases.
Figure 2. Protein structure of bilirubin oxidases.
Application of bilirubin oxidase
1. Clinical testing and treatment
Bilirubin oxidase has a wide range of uses in medicine. It is mainly used to determine the content of bilirubin and total bilirubin in serum. It is more sensitive and accurate to monitor liver and gallbladder diseases than chemical methods, thereby providing detailed pathological analysis information. In order to facilitate the determination, bilirubin oxidase has been made into a kit, which can quickly determine the bilirubin content of patients and diagnose jaundice in clinical. In addition, patients with severe jaundice need to measure creatinine in the patient's serum during clinical treatment, but they will be interfered by bilirubin in the serum during the measurement, and bilirubin oxidase can be used to eliminate the bilirubin in the serum. Interference to ensure the normal progress of creatinine measurement and improve the reliability of the value. Bilirubin oxidase is an extracellular enzyme that is not easy to isolate. In the process of isolation and purification, the nature is unstable, the enzyme activity will be greatly reduced, and the yield will be reduced. In order to overcome the above problems, we can fix the enzyme. The properties of the fixed enzyme are more stable, it can improve the catalytic efficiency of the enzyme, and it is easy to regulate and control the enzyme reaction.
2. Treatment of environmental sewage
With the rapid development of industry, more and more chemical and printing and dyeing wastewater are discharged into the environment, causing some pollution. For a long time, the methods of treating wastewater were mainly physical and chemical methods. The physical method of treating wastewater was slow and had a long cycle. With the in-depth study of enzymes, biological treatment of sewage becomes possible. Bilirubin oxidase and laccase are both polyphenol oxidases and therefore have similar properties and effects.
References
- Mosqueda, L.; et al. The Life Cycle of Bruises in Older Adults. Journal of the American Geriatrics Society. 2005, 53 (8): 1339-1343.
- Macedo LJ.; et al. Assessing electron transfer reactions and catalysis in multicopper oxidases with operando X-ray absorption spectroscopy. Nature Communications. 2020, 11 (1): 316.