-
Enzymes
-
Applications
-
Enzymes for Industrial Use
- Agriculture, Biomass, and Biofuels
- Chemical Processing
- Environment and Waste Management
- Food and Beverage Applications
- Health, Diet, and Nutrition
- Household and Daily Use
- Food & Beverage
- Animal Feed & Pet Food
- Detergent
- Textile
- Leather
- Pulp & Paper
- Agriculture & Bioenergy
- Waste Management
- Chemical & Research
- Enzymes for Research & Diagnostic Use
-
Enzymes for Industrial Use
- Biological Functions
- Catalytic Mechanism
- Featured Products
-
Applications
- Extracts
- Probiotics
- Zymogens
- Coenzymes
- Enzyme Protectant & Stabilizer
- Others
- Nanozymes
- Custom Blends
Our Products Cannot Be Used As Medicines Directly For Personal Use.


Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
MAP kinase
| Catalog | Product Name | EC No. | CAS No. | Source | Price |
|---|---|---|---|---|---|
| NATE-0443 | Mitogen activated protein kinase from rat, Recombinant | E. coli | Inquiry |
Related Reading
Mitogen-activated protein kinase (EC 2.7.11.24, MAP-Kinase) belongs to the class of transferases, is a serine/threonine-specific protein kinase that activated by extracellular stimulation. Upon activation, it catalyze the phosphorylation of substrates. The systematic name of this enzyme class is ATP:protein phosphotransferase. The first MAP-Kinase ERK1 was identified in mammals, and later studies found that MAP-Kinase only exists in eukaryotes, including plants, animals and fungi. The MAP-Kinase signaling pathway regulates gene expression, cell proliferation, differentiation and apoptosis in response to extracellular stimulation, such as mitogens, growth hormones, osmotic shock and UV radiation.
Classification
Mammals contain 14 MAP-Kinases, which are divided into two categories: conventional MAP-Kinase and atypical MAP-Kinase. Each group of conventional MAP-Kinase is composed of three kinases that act in sequence, including a MAP-Kinase (MAPK), a MAPK Kinase (MAPKK) and a MAPKK Kinase (MAPKKK). Atypical MAP-Kinases do not share the characteristics of conventional MAP-Kinases, and they do not form classical three- tiered kinase cascades.
 Figure 1. Schematic representation of conventional and atypical MAPKs. (Cargnello M, Roux P.P. 2011)
Figure 1. Schematic representation of conventional and atypical MAPKs. (Cargnello M, Roux P.P. 2011)
Structure
MAP-Kinase is a protein kinase family member. Mammals MAP-Kinases include the extracellular signal-regulated kinase (ERK) family, the c-Jun N-terminal kinase family (JNK) and the p38 kinase family. All protein kinases contain two domains, the N-terminal domain and the C-terminal domain. The N-terminal domain of ERK MAP-Kinase is formed by multiple β strands and two helices, contains the glycine-rich ATP-phosphate-binding ribbon. The C-terminal domain is mostly composed of helices, contains the the phosphorylation lip and the MAP kinase insertion, and is the locus of the P+1 site, the catalytic loop. The substrate phosphoacceptor binds to the conserved residues near the "catalytic loop", and the P+1 residue of the substrate binds to the surface pocket of the C-terminal domain. MAP-Kinases are different from other protein kinase by a 50 residues insertion (MAP kinase insertion).
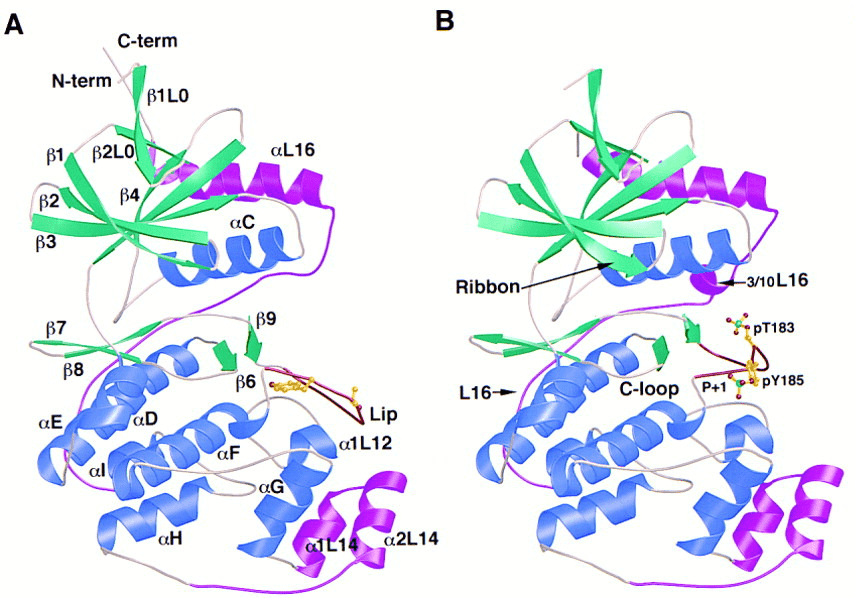 Figure 2. Structure of unphosphorylated ERK2 (A) and phosphorylated ERK2 (B). (Canagarajah B.J. 1997)
Figure 2. Structure of unphosphorylated ERK2 (A) and phosphorylated ERK2 (B). (Canagarajah B.J. 1997)
Catalytic Mechanism
Protein kinases catalyze the phosphoryl groups (PO32-) transfer from ATP to the hydroxyl group of protein substrate. MAP-Kinase is proline directed phosphotransferase, only catalyze the substrate contains proline in the P+1 site. The N-terminal domain of substrate bind to the C-terminal domain of MAP-Kinase. In the sequence of ERK MAP-Kinase, a motif called K/D/D (Lys/Asp/Asp) illustrate its catalytic function. β3-strand Lysine (K88/71) forms salt bridges with ATP. Asp166/149 in the catalytic loop is the basis of the catalysis. This Aspartate takes the proton from the -OH group of the substrate and promotes nucleophilic attacks of oxygen atoms on the γ-phosphoryl group of MgATP. The second Aspartate binds to the magnesium ion, which coordinates the α-, β-, and γ-phosphate groups of ATP.
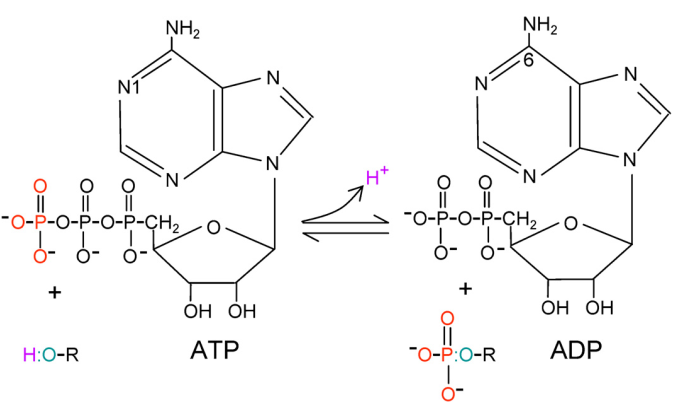 Figure 3. Catalytic mechanism of protein kinases. (Robert Roskoski Jr. 2012)
Figure 3. Catalytic mechanism of protein kinases. (Robert Roskoski Jr. 2012)
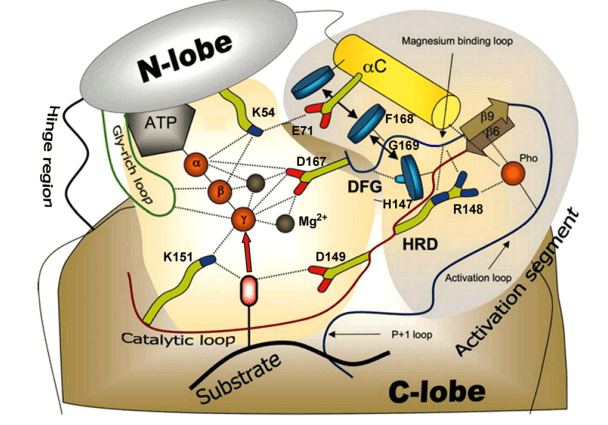 Figure 4. Catalytic mechanism of ERK MAP-Kinase. (Robert Roskoski Jr. 2012)
Figure 4. Catalytic mechanism of ERK MAP-Kinase. (Robert Roskoski Jr. 2012)
Activation and deactivation of MAP-Kinase
Activation of MAP-Kinases is regulated by two phosphorylation sites in the phosphorylation lip. All MAP-Kinases contain a Thr-Xxx-Tyr sequence that is the site of a specific MAPKK catalyzed double phosphorylation. In the ERK phosphorylation lip, MEK first mediated the phosphorylation of the Tyrosine residue. Then the Tyrosine- phosphorylated ERK separates from the MEK, and then combined with MEK again that then phosphorylates Threonine. Phosphorylation of threonine and tyrosine increased 1000-fold of ERK activity. The deactivation of MAP-Kinase is mediated by MAP-Kinase phosphatases (MKPs). The MKPs catalyze the dephosphorylation of activated MAP-Kinases following unidirectional reaction: Protein–OPO32− + HO : H → protein–OH + HO : PO32−.
Application
There are four MAP-Kinase pathways in human, The canonical MAP-Kinase pathway is composed of MAPKKK, MAPKK and downstream MAPK. MAP-Kinase pathway is critical for human cancer cell survival, dissemination, and resistance to drug therapy. MAP-Kinase pathway is a signaling node that receive input from stimulation, there are temporal and spatial regulation in cells to control the output of MAP-Kinase pathway. Normal MAP-Kinase pathway suppress tumor through induce cell senescence and ubiquitination degradation of critical proteins. Gene mutations in tumorigenesis leads to dysregulation of kinase and hyperactivation of MAP-Kinase pathway. The discovery of specific oncogenes muntations that activate the MAP-Kinase pathway have led to the development of targeted therapies for many types of tumors, ERK and MEK proteins have been successfully used as targets.
MAP-Kinase pathway is a double-edged sword. Therapeutic that inhibit elements in the MAP-Kinase pathway have achieved some success, but small molecule inhibitors targeting specific protein targets have led to the occurrence of secondary malignant tumors, so the application of this method needs to be further evaluated.
References
- Cargnello M., Roux P.P. Activation function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiology and molecular biology reviews, 2011, 75: 50-83.
- Canagarajah B.J., Khokhlatchev A., Cobb, M.H., Goldsmith E.J. Activation Mechanism of the MAP Kinase ERK2 by Dual Phosphorylation. Cell, 1997, 90: 859-869.
- Robert Roskoski Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacological Research, 2012, 66: 105-143.
- Burotto M., Chiou V.L., Lee J., Kohn E.C. The MAPK pathway across different malignancies: A new perspective. Cancer, 2014, 120(22): 3446-3456.